Group A Streptococcus
Group A Streptococcus (GAS) infections affect >700M people annually worldwide, with ~18M of these cases severe in nature, and ~600K classified as highly invasive. GAS is a human-specific pathogen responsible for an array of pathological manifestations, ranging from superficial skin and pharynx infections, to severe conditions, e.g., necrotizing fasciitis and toxic shock, and post-infection sequelae that results in rheumatic heart disease and glomerulonephritis. Severe infections have a combined fatality rate of ~30% in western countries. Currently, no GAS vaccine is available and its development has been difficult primarily due to the number of diverse GAS strains and the complications that often arise from cross-reactivity with host proteins. Antibiotics remain effective against GAS infections, however, resistant strains have been observed and present a potential threat to public health.
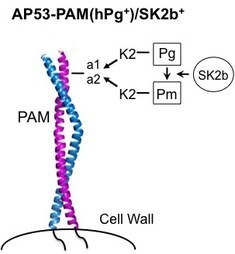
Illustration representing the interaction a1a2 region of the plasminogen binding Group A Streptococcal M-like protein (PAM) to the kringle-2 (K2) domain of host plasmin(ogen) (Pg or Pm). The secreted virulence factor streptokinase (SK2b) from PAM-containing GAS strains, like strain AP53, has evolved to preferentially activate host plasminogen bound to PAM. (Characterization of Streptokinases from Group A Streptococci Reveals a Strong Functional Relationship That Supports the Coinheritance of Plasminogen-binding M Protein and Cluster 2b Streptokinase. J. Biol. Chem. 287, 42093–103, 2012).
The more virulent strains of GAS subvert host defenses by producing factors that hijack proteins to facilitate dissemination into deep tissue at the expense of altering host vascular integrity. As an example, during infection, GAS is able to exploit components of the host fibrinolytic system, including plasminogen and fibrinogen, promoting systemic infection and permitting the organism to evade an immune response. Early work at the Keck Center has demonstrated that the M-like protein, PAM, found on the bacterial surface of some GAS strains binds to human plasminogen with extremely high affinity. In addition to binding, host plasminogen GAS secretes the potent plasminogen activator streptokinase. The combination of binding and activation of human plasminogen creates a preotolytic microenvironment around the GAS cell surface, allowing the organism to degrade host tissue, dissolve clots, subvert immune response, and colonize host tissue. The importance of human plasminogen to GAS infection has been demonstrated in a number of ways, including the observed increase in virulence in transgenic mice expressing human plasminogen.
The Keck Center uses an interdisciplinary approach focused on identifying mechanisms of host/bacteria interaction and regulatory systems important for GAS virulence. Specifically, detailed structure-function analysis of the binding of GAS surface proteins to host fibrinolytic proteins, plasminogen and fibrinogen, are being performed. In addition, a number of “omics” types of analysis are underway to identify critical genes necessary for GAS host colonization and survival. Animal models of infection are routinely used to probe the role of critical genes and understand aspects of virulence. Together these approaches help to define critical aspects of GAS infection in an effort to prevent and treat the potentially life-threatening diseases it can cause.
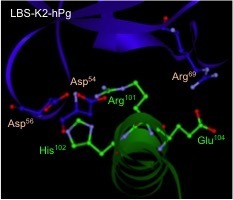
NMR structure of a peptide fragment from the a1a2 region of the Group A Streptococcal M-like protein PAM, with residues shown in green, binding to the kringle-2 domain of human plasminogen ,shown in blue. These key interactions allow the human pathogen to recruit plasminogen to the cell surface, a critical aspect of GAS virulence. (Solution structure of the complex of VEK-30 and plasminogen kringle 2. J. Struct. Biol. 169, 349–59, 2010).
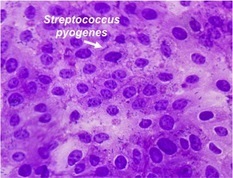
Streptococcus pyogenes adhering to human keratinocytes. A microscopic image showing a layer of human skin cells that have been infected with S. pyogenes. The small dark chains are representative of GAS adhering to host tissue, at the initiation of the infection.